Large-scale manufacturing and industry partnerships
In January 2020, Oxford had never produced more than a few thousand doses of any adenovirus-vectored vaccine. By July 2022, 3 billion doses of ChAdOx1 nCoV-19 had been produced.
Doses used in the first year of the vaccination programme saved an estimated 6 million lives1. Uniquely among the COVID-19 vaccines distributed by major multinational pharmaceutical companies, the majority of the product has been made and used in low- and middle-income countries (LMICs).
Work to scale up the manufacturing of the COVID-19 vaccine was largely distinct from early stages of the University’s clinical trial programme, which were supplied with vaccine by small-scale manufacturing runs at the University’s Clinical Biomanufacturing Facility (CBF, led by Professor Catherine Green) and Advent, an Italian manufacturer. The scale-up work was led by Dr Sandy Douglas of the Jenner Institute, with senior team members Dr Adam Ritchie and Dr Carina Joe. It received critical support from numerous external partners mentioned below. By the end of April 2020 this team had provided proof-of-concept that their process could manufacture vaccine at commercial scale, secured funding from the UK government to manufacture millions of doses for UK supply, established a network of five manufacturing sites across four countries including India and China, and begun ‘tech transfer’ to those sites. The biggest single manufacturing site was the Serum Institute of India (SII), which had joined the network in March 2020 as a result of discussions between Prof. Adrian Hill of the Jenner and Umesh Shaligram of SII. Together, these five sites went on to produce more than half of the global supply of the vaccine.
From 15 May 2020, the team entered partnership with AstraZeneca which continued process refinement, validation & tech transfer, and further extended the network of sites to span 12 countries on five continents. AstraZeneca’s manufacturing effort was led by Richard Turner (R&D) and David Hunt (operations).
Pre-pandemic adenovirus manufacturing
In the early 2000s, extensive work to develop adenovirus manufacturing technology had been undertaken by academic teams including those led by Amine Kamen (Canadian National Research Council) and Paula Alves (iBET), and by pharmaceutical companies Introgene, Crucell and Merck. These efforts converged on similar processes, in which the virus was grown in cells within a sterile vessel (‘bioreactor’), then harvested and purified using a series of filters and chromatography steps. The most successful methods were relatively complex. Janssen Vaccines, part of Johnson & Johnson, acquired Crucell and made over a million doses of its adenovirus-based Ebola vaccine over the course of a year. Their process used a specialist cell culture technique known as perfusion which few manufacturing facilities had the experience or equipment to adopt. GSK had also invested in adenovirus manufacturing after their acquisition of Okairos, but had subsequently abandoned work on the platform.
In 2019, Douglas’ group in the Jenner had published a method of manufacturing chimpanzee adenovirus-based vaccines, designed to be very simple (so it could be done by many manufacturing facilities) and so it should work robustly for new adenovirus-based vaccines, including a future vaccine against a not-yet-known disesase2. The work was done at small scale but using techniques which could be scaled up to make hundreds of thousands or millions of doses. Even if it were scaled-up massively, though, this method would not have been capable of producing enough vaccine to tackle a true pandemic.
February to May 2020: academic-led development of manufacturing process, distributed manufacturing strategy & international network
In early February 2020 it appeared that, although Oxford had the capacity to develop a COVID-19 vaccine, and clinical trials might take only a few months, manufacturing ‘at pandemic scale’ would take much longer. It was questionable whether the University would be able to develop a vaccine with real-world impact without the support of one of the large pharma companies with experience of the platform – but Johnson & Johnson had its own COVID-19 vaccine programme, GSK and Merck had abandoned work on adenoviruses years earlier, and no published method appeared suitable for manufacturing such vaccines ‘at pandemic scale’.
Dr Carina Joe, Dr Adam Ritchie, Associate Prof. Alexander (Sandy) Douglas. (C) John Cairns.
A well-timed discovery provided a critical advance. At the start of the pandemic Dr Carina Joe was the only researcher at the University working full-time on scalable production of adenovirus vectored vaccines. Douglas and Joe had agreed she would focus on means of increasing the ‘upstream productivity’ of the team’s previous process, exploring ways of improving the health of the cells which produced the virus. In January 2020, Joe had demonstrated that a simple change to the cell growth medium and ‘feeding’ regime allowed up to 10 times more vaccine to be made. She did this at a tiny scale: just 30 mL of cells (two tablespoons). If the vaccine were going to be available quickly, this needed to be repeated on a scale at least 5,000 times greater, and before it was even known if the vaccine worked. Without ‘proof-of-concept’, that it could be made at such scale, it was unlikely any major funder or pharmaceutical company would support the vaccine programme.
Assisted by the ‘contacts book’ of Professor Catherine Green (director of Oxford’s Clinical Biomanufacturing Facility, where the first small batch of vaccine was made for clinical trials), Douglas started getting in touch with the handful of biotech companies in the UK and nearby who might be able to help3. The UK government had given £67m in 2017 to build a Vaccine Manufacturing and Innovation Centre (VMIC) near Oxford. VMIC’s facility had not yet been built, but a small staff had been recruited to make preparations and VMIC’s head of process development, Jon Humphreys, became a key member of the Oxford team, and put the team in touch with a Dutch manufacturer, Halix. On 15 Feb, the UK Bioindustry Association distributed an email on behalf of the Oxford team to biological manufacturers across the country asking for support. This resulted in several offers of substantial support, notably from Pall Biotech and Cobra Biologics, whose Chief Scientific Officer Daniel Smith became a further leading member of the team.
By late February, with this support, the team had developed a plan for the new process to be tested in a large-scale ‘practice run’ at Pall Biotech’s lab in Portsmouth, while Cobra and Halix would begin preparation to transfer the process to pharmaceutical cleanrooms to produce clinical grade (‘GMP-compliant’) vaccine. Work which would normally have taken years would be compressed into a few months and, if successful, would show that the vaccine could be created at ‘pandemic scale’.
At that point, not even a single dose of clinical-grade COVID-19 vaccine had been made using a commercially-viable process. The team were doubtful about discussing, even in private with colleagues, the possibility that the process might produce hundreds of millions or even billions of doses of vaccine. No single manufacturing site would be able to deliver the scale needed and there was a risk that governments might choose to vaccinate their populations first - meaning that low- and middle-income countries (LMICs) would be at the back of the queue. It was clear multiple sites were needed (a model known as ‘distributed manufacturing’), they needed to be in multiple countries including LMICs, and immediate commitment of capacity was needed in order to avoid delay. Douglas therefore began to contact large international manufacturing sites from the first week of March 2020. Douglas & Hill, with Dr Kate Orkin & Prof Stefan Dercon (of the University’s Blavatnik School of Government, and former chief economist at the UK Department for International Development) also began to contact UK policymakers and international funders regarding the need for major ‘at risk’ financing for commercial-scale manufacturing, ahead of any clinical trial data.
This approach had never been set up at this speed or scale for any brand-new vaccine, and the majority of potential manufacturing sites had never worked on adenoviruses before. The simplicity of Oxford’s process, however, made it plausible that new sites could rapidly set it up. By mid-March 2020, five manufacturers were ready to start ‘technology transfer’ work. Three of them (Oxford Biomedica, Cobra Biologics, and Halix) would initially work on a plan to supply the UK. The other two were one of the biggest LMIC biological manufacturing contractors (Wuxi Biologics), and the biggest LMIC vaccine manufacturer (Serum Institute of India), and would provide vaccine primarily for LMICs. Other companies came on board to supply equipment and materials. The team established a co-operative consortium, co-ordinated by Dr Adam Ritchie: all sharing information, effort, and a vision of delivering a vaccine for the world as safely and quickly as possible. Consortium members began work at their own financial risk, before outside funding was confirmed, with the first cleanroom manufacturing operations (cell bank preparation) starting at Cobra Biologics in Staffordshire on 8 April 2020. The first commercial-scale (200L) manufacturing practice run was initiated at Pall Biotech in Portsmouth in early April 2020 and completed on 1 May 2020. This yielded enough vaccine for around 400,000 doses but, because it had not been performed under regulated conditions, those doses could not be used in humans.
The drug substance from one of the first even non-medical grade, large scale manufacturing runs at Pall in April 2020.
On the 30 March 2020, Douglas was contacted by Ian McCubbin, leader of a Vaccine Manufacturing Taskforce which had been set up by the UK Bioindustry Association (BIA) and was in communication with the UK government. The Taskforce requested a proposal from the University to supply the vaccine to the UK at large scale. Working with the consortium, Douglas and Ritchie prepared a proposal to make millions of doses by the end of 2020 at a cost of £65m, which was presented to the Secretary of State for Business on 13 April and accepted soon after (before the first person received the vaccine in a clinical trial).
In parallel, they proposed a more ambitious plan to the BIA team, referred to as ‘stream 2’ or ‘virtual VMIC’. This involved providing three 1000L bioreactors, all associated equipment and staff for Oxford Biomedica’s newly built but as-yet-unequipped ‘Oxbox’ manufacturing facility (less than a mile from the Jenner), using funding already allocated to equip the as-yet-unbuilt VMIC facility. This ‘virtual VMIC’ subsequently provided the majority of the UK’s supply of the vaccine.
During April, the University entered discussions first with Merck and then with AstraZeneca. Manufacturing was a key consideration in these negotiations. The University sought assurances that the chosen partner would manufacture vaccine in large volumes ‘at risk’ (before it was clear whether the vaccine would work), including in LMICs, and preferably in partnership with Serum Institute of India (which had been working since mid-March to prepare for manufacturing and had uniquely large capacity). AstraZeneca provided these assurances and undertook to supply the vaccine not-for-profit globally during the pandemic, and in perpetuity for low income countries. The University formally entered a partnership with AstraZeneca on 15 May.
In 2021, members of the University of Oxford vaccine manufacturing team, the UK Vaccine Taskforce, the BIA and a range of industry partners met in person for the first time for a celebratory event. (C) John Cairns.
May 2020 onward: partnership with AstraZeneca
AstraZeneca’s manufacturing work was led by Richard Turner (development) and Dave Hunt (operations).
Two adaptations to the 200L process which had been used in Portsmouth were made to enable it to be scaled up to 1000-4000L (i.e. the largest available bioreactors suitable for this work). Firstly, Turner’s team reduced the amount of viral ‘seed’ used in each manufacturing batch. Secondly, Douglas’ team showed that the most cumbersome step of the purification process, which was not going to be feasible at very large scale, could be eliminated completely4. Commercial-scale ‘test runs’ of this modified process at AstraZeneca, Oxford Biomedica and Serum Institute of India followed in quick succession.
In parallel with these refinements to the process, over summer and autumn 2020, AZ built on the University’s distributed manufacturing template, setting up parallel supply chains to manufacture the bulk vaccine in 12 countries on 5 continents4.
Following announcement of the efficacy of the vaccine on 23 Nov 2020, the vaccine secured its first regulatory approval (in the UK) on 30 Dec 2020. The first patient received it on 4 Jan 2021 – 358 days after publication of the virus’ genetic sequence – at Oxford’s Churchill Hospital, next door to the Jenner.
The vaccine was rapidly approved for use in more than 170 countries. A billion doses had been made by July 2021, and three billion by July 2022. Over 1.5 billion doses were made by Serum Institute alone. Independent estimates by the data analytics company Airfinity, based on a study by Imperial College London, suggested that the vaccine saved over 6 million lives in 2021, more than any other1,5.
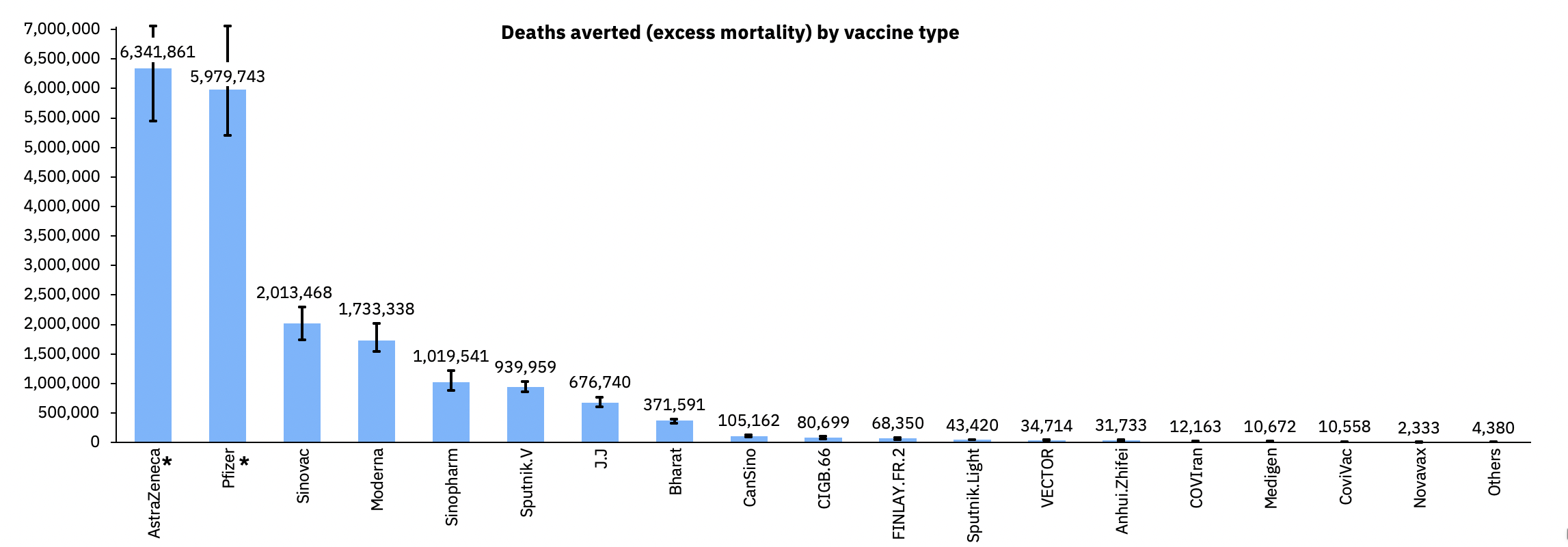
Deaths averted (excess mortality) by vaccine type.1
fURTHER INFORMATION ABOUT lARGE scALE MANUFACTURING
Innovation to deliver manufacturing and trials
Blueprint for a vaccine in 100 days
References
1. Airfinity. AstraZeneca and Pfizer/BioNTech saved over 12 million lives in the first year of vaccination. 13/7/22 2022. https://www.airfinity.com/insights/astrazeneca-and-pfizer-biontech-saved-over-12-million-lives-in-the-first (accessed 12/8/22).
2. Fedosyuk S, Merritt T, Peralta-Alvarez MP, et al. Simian adenovirus vector production for early-phase clinical trials: A simple method applicable to multiple serotypes and using entirely disposable product-contact components. Vaccine 2019.
3. Pike H. The Oxford miracle: making enough COVID-19 vaccine to supply the world. The Pharmaceutical Journal. 2021 15/4/21.
4. Joe CCD, Jiang J, Linke T, et al. Manufacturing a chimpanzee adenovirus-vectored SARS-CoV-2 vaccine to meet global needs. Biotechnol Bioeng 2021.
5. Watson OJ, Barnsley G, Toor J, Hogan AB, Winskill P, Ghani AC. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. The Lancet Infectious Diseases.




